18 Predict Which of the Following Has a Covalent Bond
1 single bond and 4 double bonds. Example Exercise 121Bond Predictions.

Covalent Bond Examples Formation Properties What Is A Covalent Bond Video Lesson Transcript Study Com
Dinitrogen Teroxide has a covalent bond because both nitrogen and oxygen are nonmetals meaning that they both have a negative charge.

. As the valency of germanium is 4 it can share 4 electrons of neighboring atom to complete the octet. State the oxidation state of the metal and the total valence electron count of the following species. Draw an electron dot structure for each.
Naci O CO2 KBr O Mg OH2. Use partial charges to show that a bond is polar. Draw the electron dot structure of the hydroxide ion OH-.
A non metal can co. It follows that NH3has covalentbonds. 2 Predict which of the following has a covalent bond.
Up to 24 cash back 2. Predict which of the following has an ionic bond. 12 Predict which of the following has a covalent bond.
According to the valence bond theory when a covalent bond is formed between two reacting atoms the potential energy of the system becomes_____ 1 negative 2 positive 3 minimum 4 maximum. The electrons in a polar bond tend to spend more time around the least electronegative element. The strongest covalent bond is formed by the overlap of If considering for same shell_____ 1 s and p orbitals 2 s and s orbitals.
Hence Ge has covalent bond. Explain the difference between a nonpolar covalent bond a polar covalent bond and an ionic bond. Who are the experts.
State the oxidation state of the metal and the total valence electron count of. Students should be able to. WCN 8 3 Ans.
Because the ions arent oppositely charged it couldnt be a ionic bond so I knew it had to be a covalent bond based on the charge and that nonmetals are commonly bonded together in. C one atom must be a H atom. Chemistry questions and answers.
0 and 18 5. None of the above. Cl 2 CO b.
This problem has been solved. Chemistry questions and answers. 3 and 14 2.
Two or more nonmetal atoms are attracted in covalent bonds. A metal ion and nonmetal ion are attracted in ionic bonds. Answered expert verified.
Predict which of the following has a covalent bond refer to Periodic Table. 5 and 17 4. N to N 30-3.
A CoOB NO C ZnO D all of the above E none of the above. Subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. 20 A CH4 B NH3 C PH3 D ll of the above E none of the above.
This produces a polar covalent bond and may cause a molecule to have a permanent dipole. Fe 2 CO 9 Ans. 0 and 18 Self Assessment test.
Experts are tested by Chegg as specialists in their subject area. HCl KCl NaCl all of the above none of the above Draw the structural formula for the chloride ion ClO_2- and state the type of bonds in a chloride ion. H 2 O 2 b.
D both atoms in the bond must be metals. View the full answer. D all of the above.
All of the above e. We usually call those above 18 ionic but those very close to 18 or 19 still are polar covalent although more ionic than covalent. All of these e.
Electronegativity difference of C and C. Dec 3 2012. E none of the above 13 Predict which of the following has a covalent bond.
Draw the electron dot structures for sulfate SO 4 2- and carbonate CO 3 2. Previous question Next question. K-Cl 082 - 316 234.
The correct option is D all of the above. When electronegativity difference is 18 or greater than the bond formed is ionic in nature. D all of the above.
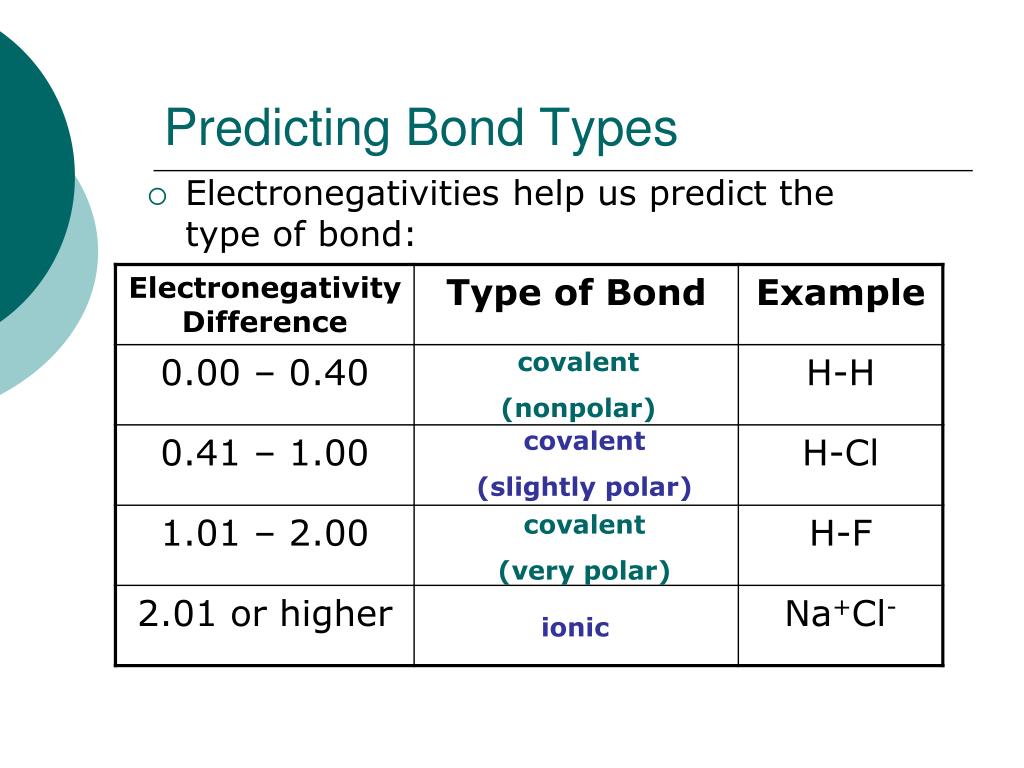
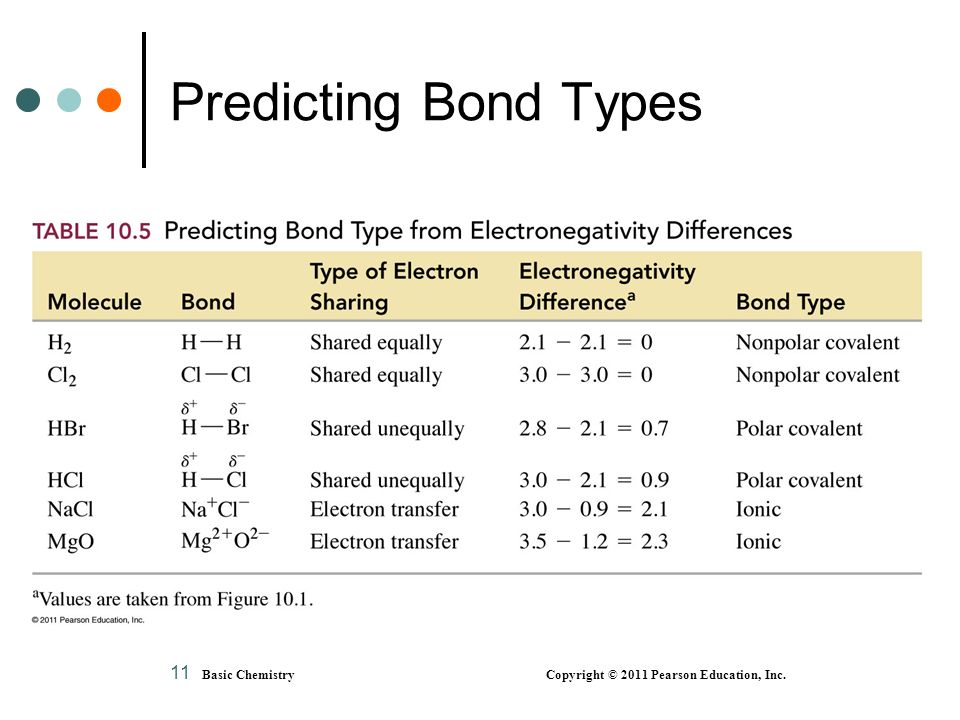
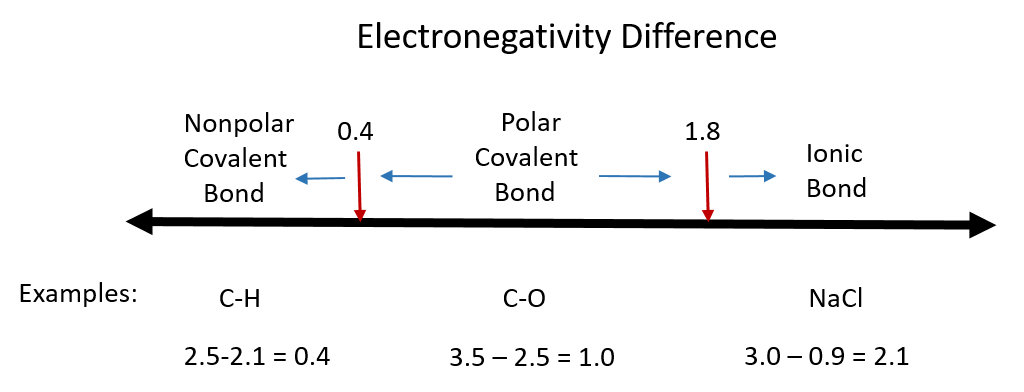
14 Predict which of the following has a covalent bond. Differences in element electronegativities may be used to predict the type of bonding ionic or covalent in a substance. Between zero and 18 is olar covalent.
Electronegativity difference of the given molecules is as follows. Nature of covalent and dative covalent bonds. QUESTION 9 Predict which of the following has a covalent bond.
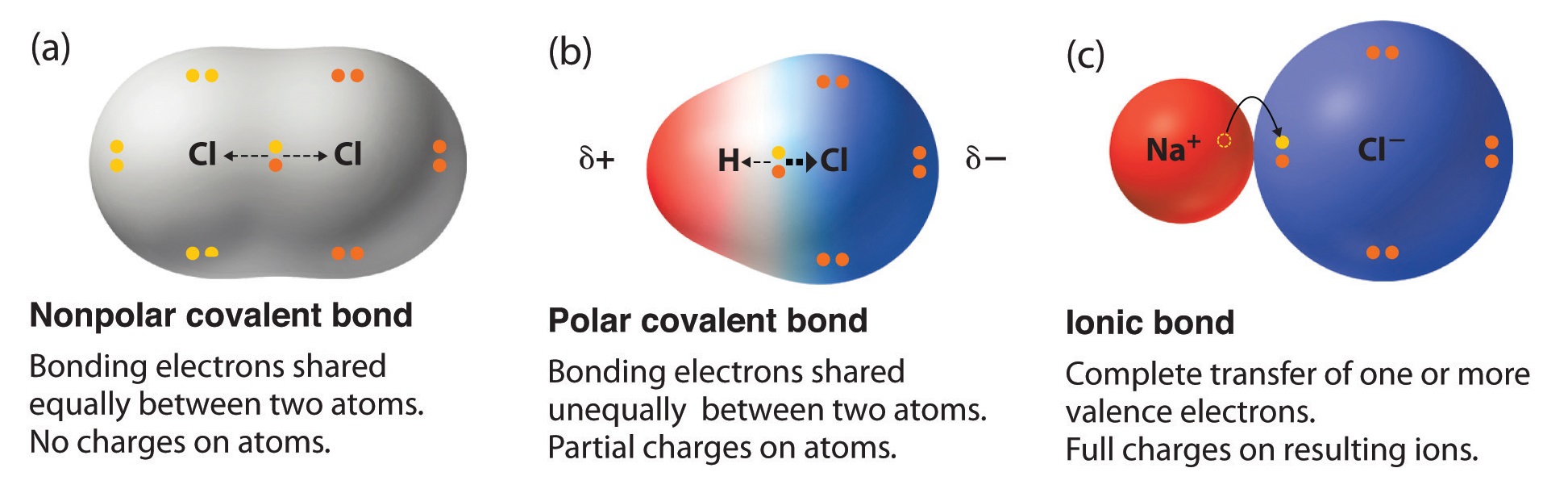
The electron distribution in a covalent bond between elements with different electronegativities will be unsymmetrical. We review their content and use your feedback to keep the quality high. Predict which of the following has a covalent bond.
Draw the structural formula for nitrogen N2 and state the type of bond in a nitrogen molecule. VC 2 O 4 3 3. Si-O 190 - 344 154.
1 single bond and 1 double bond 2 single bonds 1 single bond and 1 triple bond none of the above Predict. Nonmetals are also commonly covalent bonds. Predict which of the following has a covalent bond.
PVnRT TPVnR 20c 273 293 025000821 0020525293 0333 87987 87987 x 0020525 1805 atm. Which of the following describes the share of a non-bonding electron pair on a N molecule with an O atom resulting in a nitrous oxide molecule. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 15. K 2 S f. Take the difference of electronegativity between the two atoms.
A Ammonia contains the nonmetals nitrogen and hydrogen. 3 and 16 3. In a polar covalent bond A both atoms in the bond have the same level of electronegativity.
B one atom in the bond must have higher electronegativity than the other atom. When electronegativity difference is greater than 04 and less than 18 then bond between the two atoms is a polar covalent bond. Draw the electron dot structure of the polyatomic boron tetrafluoride anion BF 4-.
E none of the above. Draw the structural formula for ethylene C2H4 and state the type of bonds in an ethylene molecule. The following molecules have single covalent bonds.
The larger the differences in electronegativity between two bonded atoms the more polar the bond. If delta EN electronegativity 18 or 19 that is about 50 ionic50 covalent. None of the above.
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. 20 Predict which of the following has an covalent bond. B Magnesium nitride contains a metal Mg and a nonmetal N.
Predict which of the following compounds are ionic and which are covalent based on the location of their constituent atoms in the periodic table. D all of the above.
Ppt Covalent Bonds Powerpoint Presentation Free Download Id 2610463
8 7 Bond Polarity And Electronegativity Chi Chemistry Libretexts
Chapter 10 Structures Of Solids And Liquids Ppt Download
How To Predict Whether A Bond Is Ionic And Covalent

Polar Vs Nonpolar Covalent Bonds Examples What Are Polar Nonpolar Covalent Bonds Video Lesson Transcript Study Com
Ch105 Chapter 4 The Shape And Characteristics Of Compounds Chemistry

Factors Influencing The Formation Of Ionic Bonds Video Lesson Transcript Study Com

8 7 Bond Polarity And Electronegativity Chi Chemistry Libretexts

Bonding General Rule Of Thumb Metal Nonmetal Ionic Ppt Download
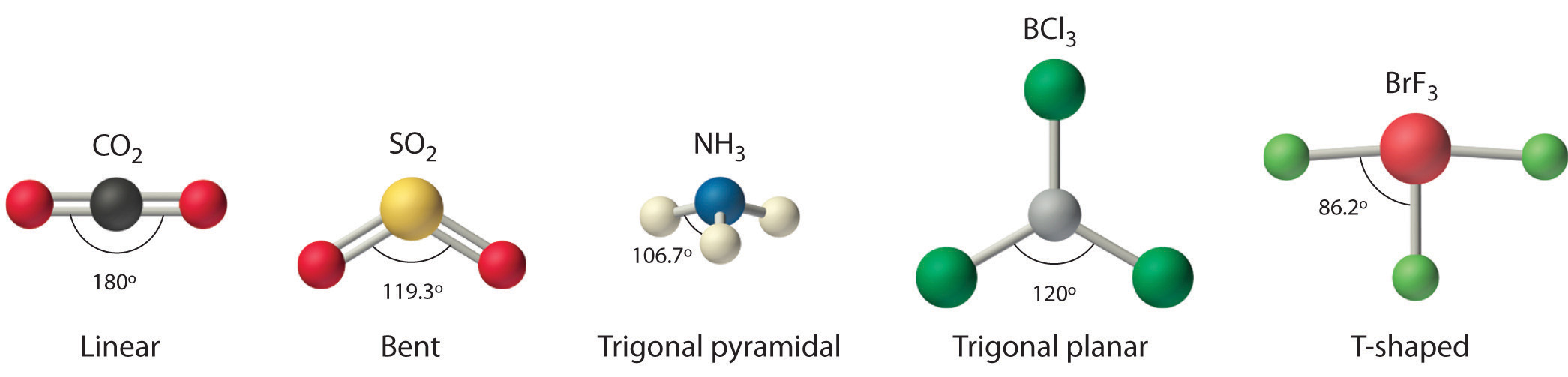
Molecular Geometry And Covalent Bonding Models

Single Covalent Bond Molecule Examples What Is A Single Bond Video Lesson Transcript Study Com
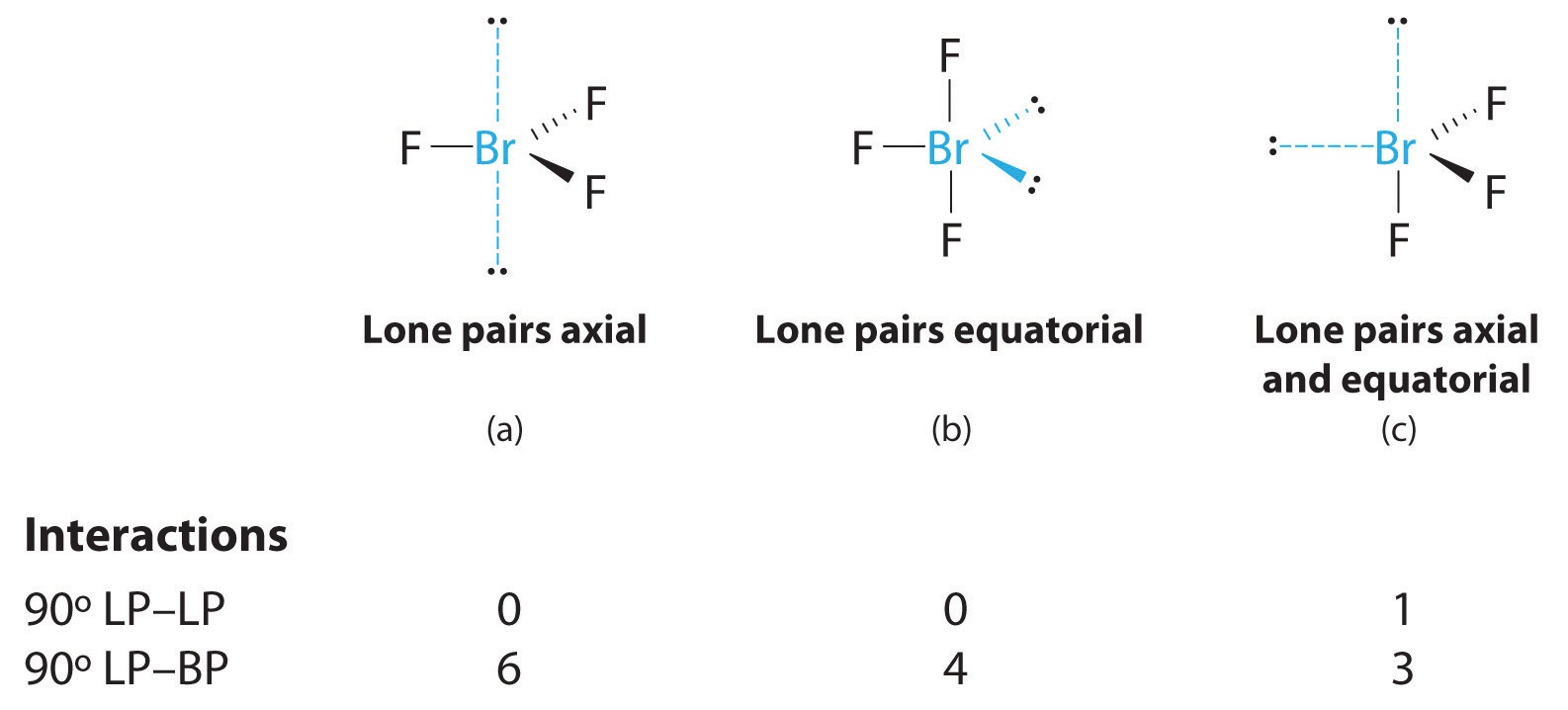
Molecular Geometry And Covalent Bonding Models

Chapter 5 Compounds And Their Bonds Ppt Video Online Download

Chemistry Life The Universe And Everything
Covalent Bonds Video Khan Academy


Comments
Post a Comment